SocraMate eCRF and SocraMia eDiary A Clinical Trial Dream Team
Every good story has its heroes. Ours just happen to work in clinical research. Meet Investigator Isabella and Data Manager Damian, and discover what SocraMate means in their everyday work.
See how SocraMia helped participant Paul and his grandmother Paula build a daily routine, and explore what software engineer Stephen had in mind when the first software idea was born.
A tale of eCRF mastery, told by people who bring it to life.
“Harmony in Health - A Tale in Eight Chapters.”
Discover the full storyPrologue
A new era had begun when SocraMate and SocraMia entered the stage of clinical research in Socraland and beyond, across several companies and institutions.
Eight people. Eight routines. One EDC Software that would change everything. Suddenly, work felt easy, almost weightless. Simon caught himself whistling while entering data.
And this is where their story begins.

"SocraMate speeds up my day"
Simon, Study Nurse

Simon, a dedicated study nurse, was immediately impressed by the SocraMate eCRF system. What stood out to Simon right away was the system's remarkable speed and intuitive design.
"I really like the conversational query view. It's super easy to use, and everything shows up right where it belongs - attached to the relevant data field. And finally, I can do my data entry without constantly clicking save and edit buttons. That alone saves me a lot of time every day," he explains.
The thoughtful way the system supports workflows in clinical research is highly appreciated by Simon. "You can tell the developers really understand what it's like to work at a trial site," he says. "Everything is where you need it, and it just makes sense."
Simon also finds the edit checks during data entry very helpful, and the integrated IWRS technology simplifies randomisation and IMP management, making SocraMate an invaluable tool in his daily routine.
"It's definitely the most advanced tool I've ever used in my career. SocraMate is my partner at work," he adds with a smile.
Curious about fast and convenient Data Entry?
Data Entry made lightning fast
Direct access to entry fields eliminates the need for "Edit" buttons or the waiting for the Audit Trail to load. Auto-save within milliseconds ensures data is instantly captured upon leaving a field without manual saving, i.e. no necessity for "Save" buttons.
Patient data can either be entered from top to bottom during a visit without interruptions. Alternatively, moving between forms using innovative navigation is just as fast and comfortable, with no delays.
Filtering and sorting patient lists happens in a fraction of a second. Even with large amounts of data, SocraMate remains incredibly fast - the average page load time is 50ms.
The system also provides immediate feedback whenever edit checks are triggered.
The conversational query view allows users to initiate queries, correct entries, and track dialogues across different roles - all at one glance and easily retrievable via a field-based audit trail.
"The system that puts my patients first"
Isabella, Investigator

"My focus is clearly on my patients." For Isabella, that's not just a statement - it's the reality of her everyday work. As an investigator, she has a full desk, a lot of responsibility, and limited time. Between patient visits and documentation, she needs tools that work with her, not against her.
She relies on a fast and intuitive system that gives her a clear overview of all entered data and allows her to review and approve it quickly. "And that's where SocraMate comes in as my support! The automated notifications and alerts save me a lot of time and keep me updated."
MedDRA coding has even become work she enjoys. While using the coding tool for term selection,
she says with a smile:
"You can tell the system was developed with users in mind - it combines regulatory compliance and everyday usability."
Isabella is well aware of the regulatory challenges that come with an EDC system, such as timely data release, and appreciates how smartly these are implemented.
With SocraMate by her side, Isabella can focus on what matters most: her patients.
Curious how Isabella handles MedDRA Coding?
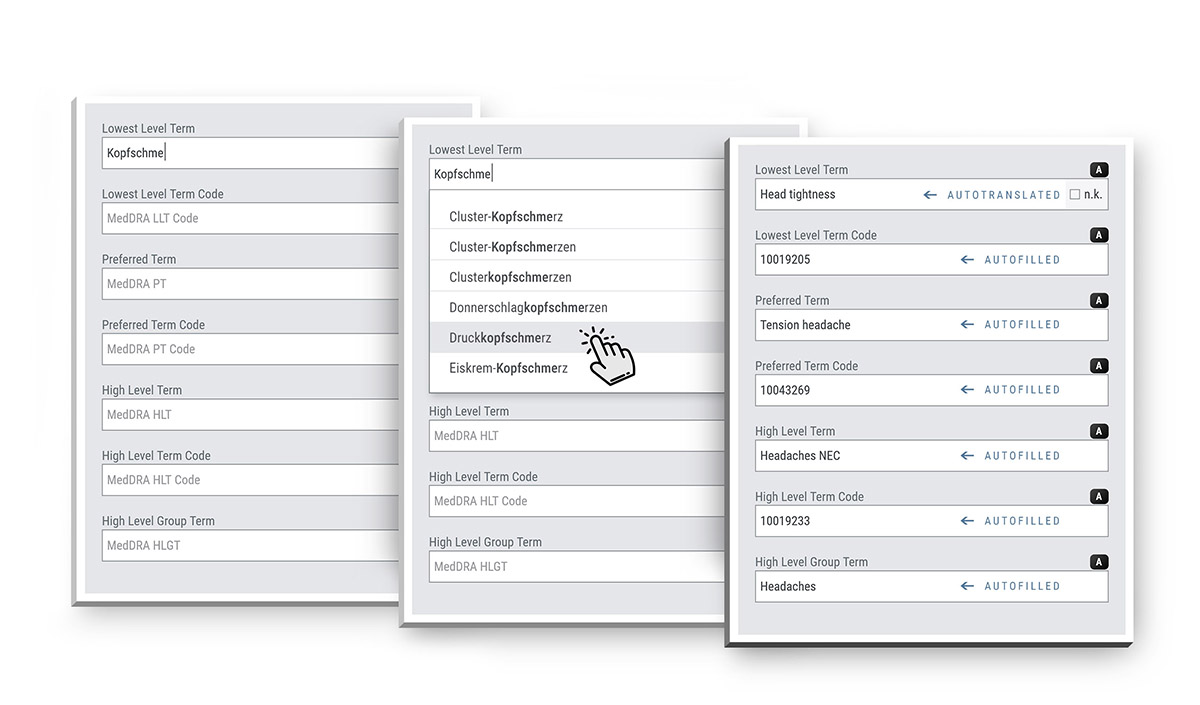
MedDRA Coding made easy
The MedDRA Coding Tool allows users to search for terms in any language supported by MedDRA. Coding can be performed on-the-fly during data entry, directly within the corresponding forms. Term selection is supported through automatic completion of codes and terms based on the selected Lowest Level Term (LLT) - fully embedded in the data entry routine and without switching views or interrupting the workflow.
To ensure consistency, an advanced autocoder tool serves as a coding assistant by combining automatic coding with a study-specific library to manage complex terms.
SocraMate supports the use of different MedDRA versions and languages across multiple trials, and enables data to be stored in any configurable alternative language offered by MedDRA.
The maintenance of MedDRA versions is simple and user-friendly.
"Easy to use, privacy built-in"
Paul and Paula, Trial participants

Paul is a typical young trial participant, always online and on the move. Yet he is highly compliant, thanks to the excellent eDiary SocraMia. No matter if others are chatting, shopping or enjoying music around him, he easily enters his data into the eDiary anytime.
"With SocraMia, filling in my eDiary is a piece of cake," says Paul. "Even my grandmother said it was intuitive and easy to use.
Paul's 76-year-old Spanish grandmother, Paula, agrees: "Yes, at first, I was reluctant, but now I can't wait to use it in the trial I signed up for. It's really fun to use SocraMia and I love that I can use it entirely in Spanish! What I appreciate most is that I don't have to use an email address. Honestly, I sometimes get the impression that I'm more aware of data protection than the younger generation."
Paul laughs. "You might be right, Grandma! We could all learn something from you."
Meanwhile, Paul gets a short call from Study Nurse Simon, who has spotted a small inconsistency in his diary data. But thanks to the smooth connection between SocraMate and SocraMia they clear it up in seconds and even share a laugh about his entry "slept 25 hours".
Curious how Paul and Paula keep perfect compliance?
SocraMia eDiary - Real-time participant data without compromising privacy
No additional software is required to use the eDiary. Participants have the flexibility to enter their data using their own smartphone, tablet, or any other device, without needing to disclose personal details such as email addresses or phone numbers. SocraMia incorporates an password recovery system, eliminating the need to store any non-pseudonymised data.
The data collection process, designed with clarity and user-friendliness in mind, fits naturally into participants' daily routines, especially when accompanied by the helpful reminder function and multiple language options.
As SocraMia eDiary is fully integrated into SocraMate, trial staff have access to real-time diary data, enabling them to perform compliance checks, view ad-hoc statistics, and intervene quickly in case of any problems. This ensures high quality of participant data and a smooth trial process for everyone involved.
"Protocol Deviations? Sorted!"
Cori, Clinical Research Associate

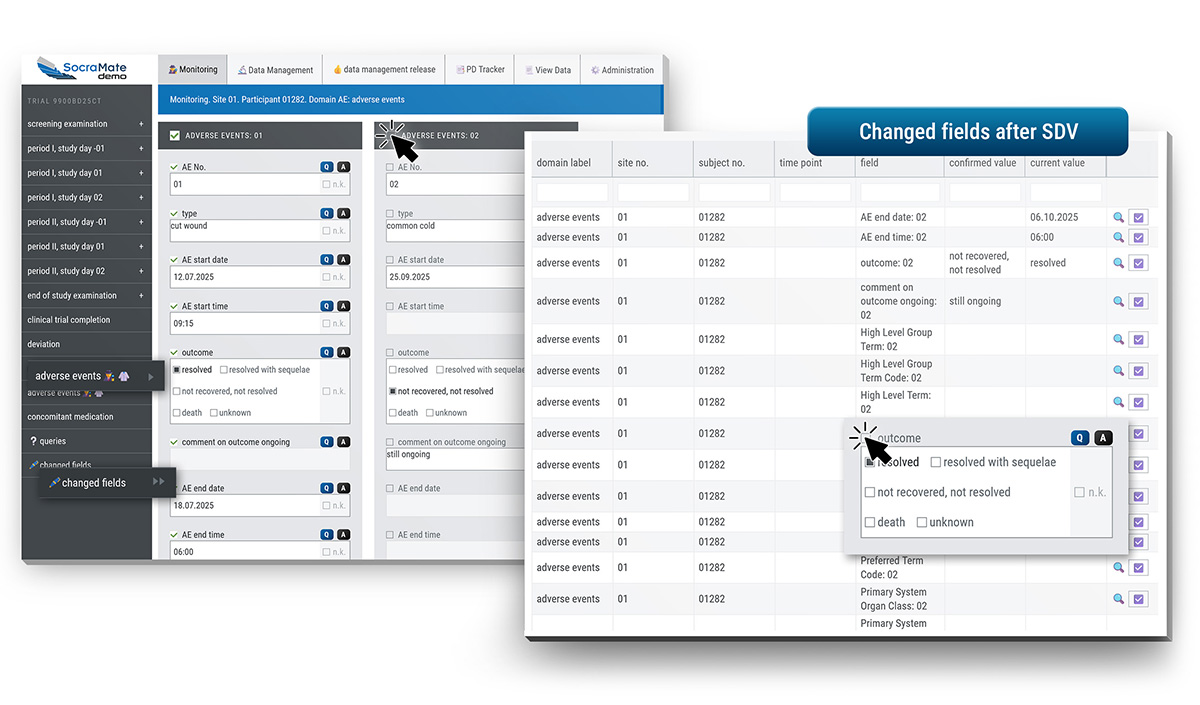
Using SocraMate, CRA Cori ensures data integrity. "I particularly benefit from the system's flexibility during SDV," Cori explains. "It lets me release individual parameters or entire sections according to my preferences or study needs. And thanks to the distinctive icons I can see that the Medical Monitor has already done an excellent job on the Adverse Events. Data quality is higher in SocraMate than in any other system I've worked with."
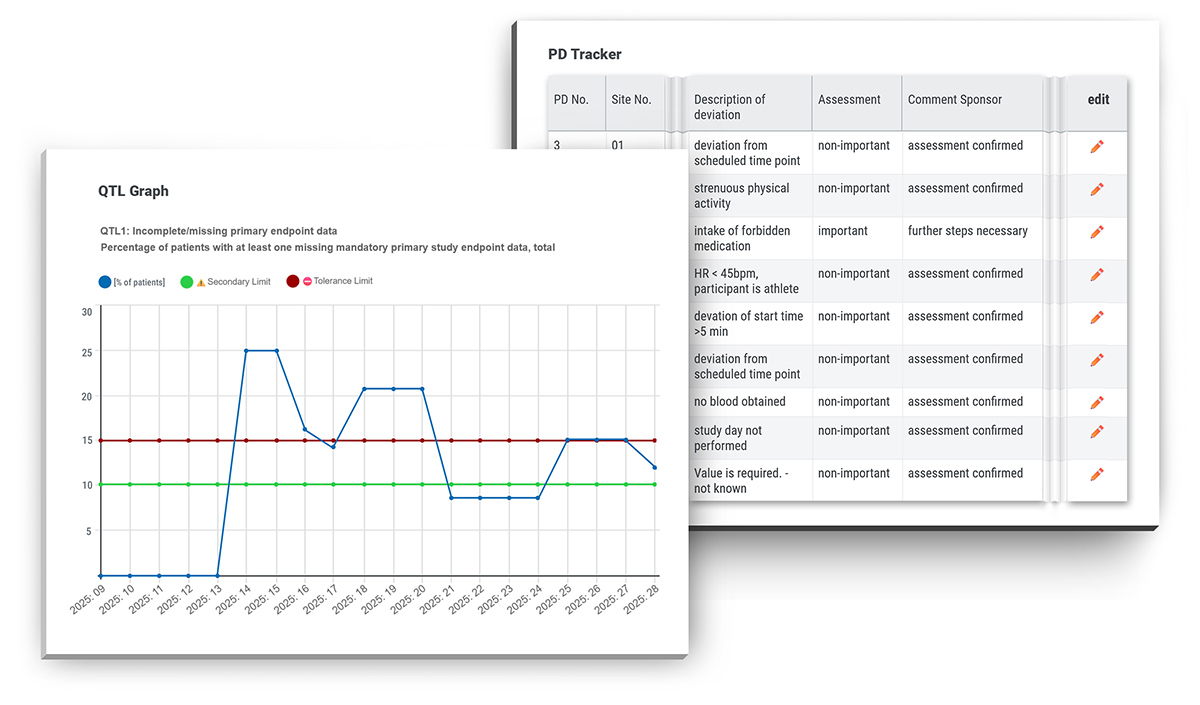
During her monitoring activities Cori often makes use of the real-time visualisations and summary statistics, combined with alerts tailored to specific Quality Tolerance Limits (QTLs) and Key Risk Indicators (KRIs). These customisable features make it easy for her to spot hidden problems or systematic issues.
"But the very best thing about SocraMate is the PD tracker. This feature has saved me hours of work so far. I love this system and would prefer to work with it in every clinical trial," Cori enthuses.
Curious how Cori masters PDs?
Advanced handling of Protocol Deviations
The Protocol Deviation Tracker (PD Tracker) embedded in SocraMate enables comprehensive and efficient documentation, tracking, and assessment of all protocol deviations throughout the course of a clinical trial.
Deviations can be added to the PD Tracker via one-click auto-fill based on real-time eCRF data such as auto queries or other trial-specific eCRF sources.
In addition, protocol deviations arising from sources outside the eCRF scope (e.g. monitoring reports) can be entered manually using a well-designed, user-friendly entry form.
Rights to enter and edit protocol deviations can be assigned to any user role (trial staff, CRA, CDM, sponsor). The PD Tracker also includes powerful filtering, sorting and export functionalities.
All protocol deviations are conveniently accessible to all relevant parties. Thanks to fine-grained access rights on column level, sensitive information such as treatment details can be selectively displayed or hidden based on user roles.
Everything is, of course, logged in the audit trail. The PD Tracker also offers a flexible setup – its features and details can be tailored to the specific needs of any project.
Altogether, the PD Tracker provides intuitive workflows to efficiently add and assess protocol deviations - at a glance, in one place, and in real time.
"SocraMate is built the way I like to work"
Damian, Data Manager

Damian, a Clinical Data Manager, is incredibly quick at setting up eCRFs, thanks to the simple and well-defined process. "I love bringing protocol specifications to life by designing a user-friendly eCRF," he says.
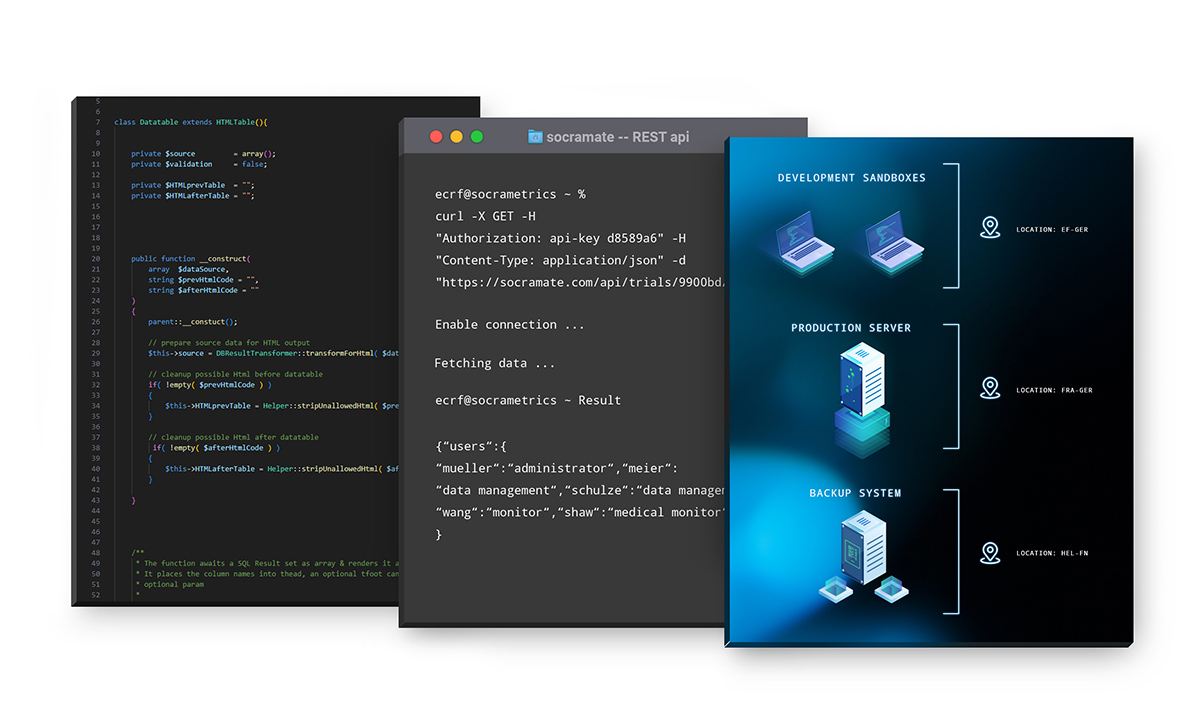
He's especially glad he doesn't have to rely on endless drag-and-drop fields. “That would be pure pain for large studies. I can do everything with point & click. But to really unleash the beast, I use SQL. That's exactly my cup of tea,” he says with a grin.
Just yesterday afternoon, Project Manager Patricia dropped a surprise: a set of changes that needed to be implemented before Go-Live. "Usually, last-minute changes like this would mean hours of extra work," Damian says, "but with SocraMate, I got it done in no time and still met today's deadline."
Even changes after Go-Live are quickly implemented and communicated to the whole team via SocraMate news box.
During the trial, he keeps a close eye on the eCRF and eDiary data, easily spotting any outliers using the summary statistics and real-time figures.
"And when it's time for the Data Review Meeting, I feel way more relaxed than in the days before SocraMate, thanks to the PD Tracker. With this dream team SocraMate and SocraMia, it's not just Data Management. It's Data Mastery," he concludes.
Curious how Damian turns SQL into study success?
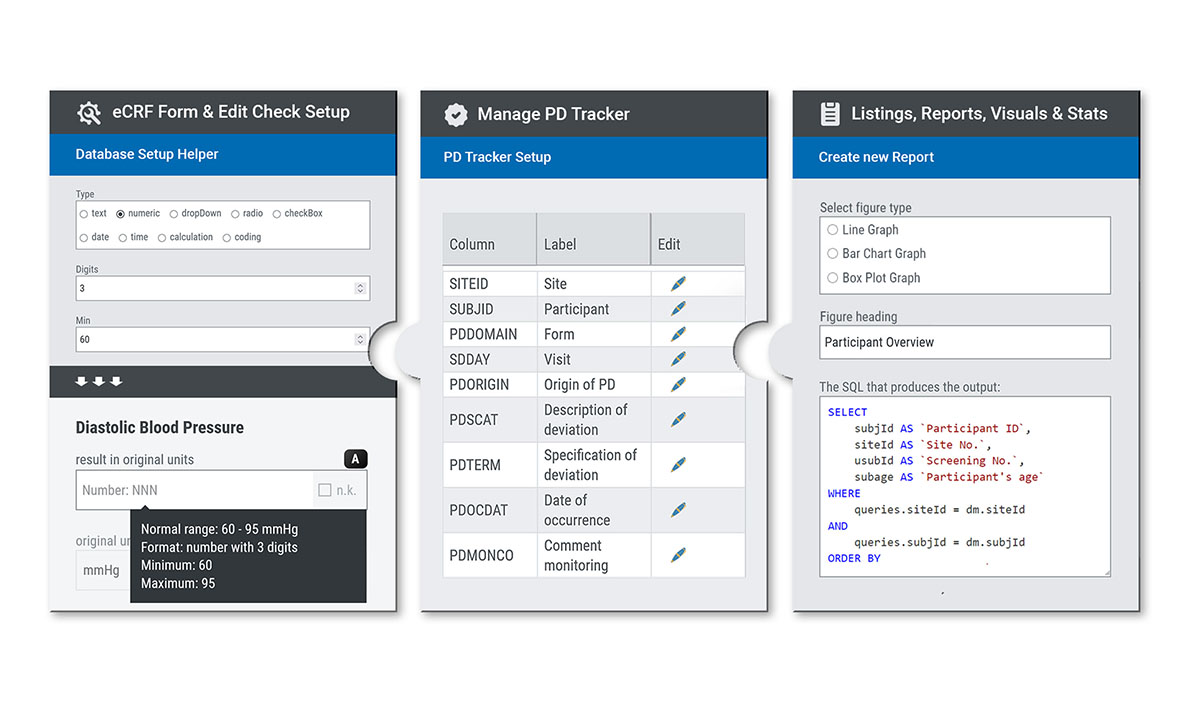
Set up smarter: easy, fast, flexible
The setup process in SocraMate is designed with future data usability and analysis in mind. Following CDASH standards, the eCRF is built with a clear vision of an optimised structure to support further data processing. So, you are in control of what comes out of your eCRF: structured, CDISC-compliant and ready-to-use data.
Data from external sources (e.g. laboratory values) can be imported into the eCRF with just a few clicks by using the REST API feature.
Every trial can be set up using the point & click interface. But with just a bit of SQL knowledge, you can unlock SocraMate's full power and efficiency: from entry forms and edit checks to data listings, reports, statistics, the Protocol Deviation Tracker, embedded QTLs and KRIs, and graphical visualisations. This flexible and modular approach enables the system to adapt to the specific requirements of each trial, no matter how complex.
Changes after Go-Live (e.g. from protocol amendments) can be implemented quickly and efficiently at any point during an active trial with no system downtime to keep workflows uninterrupted for everyone involved. A news box keeps the entire study team informed of key information and the latest changes throughout the trial.
"One click gives me the full picture"

Patricia, Project Manager
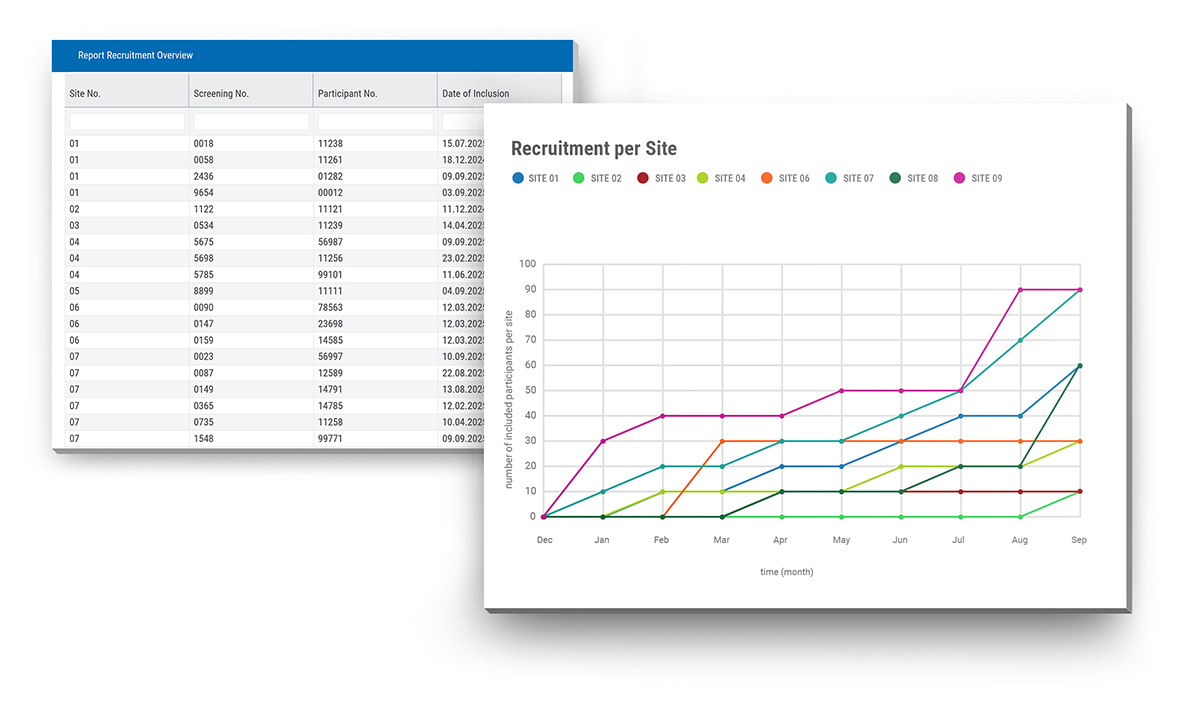
For Patricia, a dedicated Project Manager responsible for several trials in SocraMate, quick access to visit overview and recruitment status has become an invaluable asset in her daily work. In the days before SocraMate, she used to create recruitment status graphs manually.
"It's such a relief that this is now a thing of the past", Patricia sighs. "Now, one click is all it takes to see real-time recruitment visuals." If protocol amendments or other changes are necessary, a quick message to Data Manager Damian is all it takes and the updates are implemented in no time while the trial is running. "Every eCRF user is made aware of the changes upon login by the central SocraMate news box. That's really helpful for communication with the sites," Patricia adds.
Above all, Patricia appreciates the level of oversight SocraMate provides. "At a glance, I can see how many queries are still open, whether all Adverse Events have been MedDRA coded, if all protocol deviations have been assessed, and where we stand with monitoring," she explains.
"It makes everything so easy to follow," she says with a smile, knowing that SocraMate is a reliable partner in her work.
Curious how Patricia tracks recruitment in one click?
From data to visuals
Visual modules can be tailored to the needs and complexity of each clinical trial. With just a few clicks, users can access real-time visualisations which offer clear insights into key parameters such as trial progress, recruitment status, dropout rate, lab values, etc. This enables continuous and proactive oversight at all times, supports early identification of irregularities or emerging patterns in the data, and helps prevent issues before they escalate.
The audit trail is both comprehensive and performance-optimised. Data can be accessed, sorted, filtered, and exported within seconds.
Following trial selection, users are guided to a project overview which includes visual status indicators. These intuitive icons clearly reflect the status of each individual participant.
Crucial information, such as protocol amendments and their implications for trial conduct and data entry, is displayed prominently in the participant overview, securing that updates are communicated in real time and easily visible to all users.
My decisions are backed by real-time insights.
Susan, Sponsor

Susan, who works for a pharmaceutical company, already knows what's coming when Project Manager Patricia calls. SocraMate had notified her earlier that a QTL for missing primary endpoint data had been reached. "I got the notification immediately," Susan says. "It's very good to know that SocraMate keeps me informed about every relevant change. Nothing slips through. That gives me peace of mind."
She smiles as she describes how the system supports her in fulfilling her sponsor oversight duties: "These project-tailored, built-in QTLs and KRIs combined with statistics, visualisations and notifications are a solid foundation for reliable oversight."
And it saves time, too.
"Especially in Data Review Meetings," she adds. "The PD Tracker keeps everyone updated, with assessment and justifications in one place. That's perfect for keeping things clear and efficient."
What Susan also values is the outstanding support: "When we had special requirements for one of our trials, we could speak directly with the lead developer. We submitted a feature request - and it was implemented. With SocraMetrics, you're not just a number. You're truly listened to and supported."
Susan knows her project is in good hands with SocraMate.
Curious how Susan keeps full oversight?
Comprehensive Sponsor Oversight for modern clinical trials
With SocraMate, Sponsor Oversight is not just an add-on - it is a core component, consistently integrated across all essential system modules from day one.
The PD-Tracker lays the foundation of timely and traceable documentation of protocol deviations. It provides a real-time, central point of reference for all parties involved and enables the continuous, structured and standardised assessment of new protocol deviations in accordance with ICH Guideline E6(R3).
For full transparency and control, graphical visualisations and statistics of real-time data are available at the push of a button. This strengthens oversight and supports faster, more informed decision-making.
When predefined QTLs and/or KRIs are met, SocraMate automatically notifies the relevant users via alerts and email notifications.
This is what Sponsor Oversight in modern clinical trials should look like.
"I build tools that work for people."
Stephen, Software Engineer

Stephen keeps a steady pace on his office treadmill. "You know," the software developer says as he walks, "I've had enough of watching my data manager colleagues struggle with the limitations of other EDC systems. When I saw their frustration, I knew I could build something better."
The idea had struck him one quiet weekend. Years of working with people in different roles across the clinical trial lifecycle had sharpened his eye for what truly matters. "A system built from the user's perspective. That was my vision!" And he always had the full trust and support of Julianne, "the best boss in the world!"
"Regulations and Guidelines? Covered. User-Experience? Always on my radar. Cybersecurity - don't get me started," Stephen says with a grin. "It's ignored far too often in favour of fancy design, and I'm definitely not letting that happen with SocraMate."
From day one, Stephen has insisted on direct contact with customers. "People deserve real answers from real humans," he tells his growing team. "No chatbots, no automated replies that do everything but answer the question. And when a client has a good idea, it goes straight on our development wishlist."
His teammate Linda smiles. "So basically, you're the mastermind behind SocraMate?"
Stephen shakes his head. "No, not the mastermind. Just someone who wants to do a good job and help others do theirs."
Curious how Stephen turned EDC frustration into innovation?
Software development with system performance and user needs in mind
"Modern" software engineering often relies on a large number of external components. Although these are intended to accelerate the development process, they often come at the expense of performance and security. Numerous examples show that such complex systems can fail when even the smallest changes are made to the basic components.
A clear indicator of this is the perceived performance of modern software. Despite steadily increasing computing power in recent decades, many applications still feel very slow.
SocraMetrics takes a different approach: by avoiding external components, we achieve a massive reduction in complexity. This is why our EDC SocraMate is as fast and as secure as one should expect today.
What might initially sound like a contradiction proves to be a clear advantage in practice. Development times have been significantly reduced. New features or changed regulatory requirements are quickly implemented and thoroughly tested at SocraMetrics.
Without the burden of external components and thanks to very lean workflows, we were able to efficiently develop a powerful product – made by people, for people.
Epilogue
Years later, when SocraMate had supported hundreds of successful trials, one truth remained clear: work had become so much easier. Not because the trials grew less complex (on the contrary), but because people worked closer together.
What began as just another EDC tool had turned into a true partner. Different tasks, different roles, different needs, sometimes worlds apart in distance, and still the same flow, the same language, the same solid framework they could all rely on.
One system. One team. One SocraMate.